Power Supply
As a power supply, our car uses two aluminum-air batteries, one for motor driver and one for Arduino. These consist of an aluminum plate as anode, a cotton pad (wetted with KOH solution) as a separator, and a steel mesh coated with the oxygen capture mixture as a cathode. To make many cells fit and work as robust as possible, we designed a compressible box. Our battery operates with the following redox reaction:
The anode oxidation half-reaction:
Al + 3OH- → Al(OH)3 + 3e- + 2.31V
The cathode reduction half-reaction:
𝑂2 + 2𝐻2𝑂 + 4𝑒- → 4𝑂𝐻- + 0.40V
The total reaction:
4𝐴𝑙 + 3𝑂2 + 6𝐻2𝑂 → 4𝐴𝑙(𝑂𝐻)3 + 2.71V

Stopping Mechanism
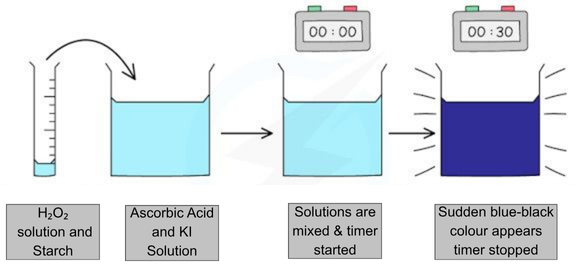
As our stopping mechanism, the RGB sensor detects color change in the reaction, which is designed to change color at a specific time by arranging the Lugol concentration. The process from the RGB sensor detecting the color shift to the car stopping works as follows: as the car moves, the RGB sensor continuously measures the color values. Once the sensor identifies the desired color range, the Arduino signals the driver to stop the motor. This ensures the car halts when the iodine clock reaction reaches the intended color change.
2H+ + 2I- + H2O2 → I2 + 2H2O
I2 + C6H8O6 → 2H+ + 2I- + C6H6O6
I- + I2 ⇌ I3-
I3- + Amylose (Starch) ⇌ Blue Complex
Mechanics and Design
Our car BeeMo is designed to consist of two floors. The lower floor is a plate with 2 axles and 4 wheels. This plate is made of plexiglass. The motor, arduino, motor driver, regulator, the container where the Iodine-Clock reaction takes place, and the RGB sensor are located on this lower plate. The valve mounted on the Iodine-Clock reaction container on the lower floor reaches the second floor via a hole. Our batteries are located on the second floor.

